RNA binding proteome (RBPome)
| PID | Title | Method |
Time | Author | Doi |
|---|
| 30607034 | Comprehensive identification of RNA protein interactions in any organism using orthogonal organic phase separation (OOPS) | OOPS & HEK293 | 2019 Jan 3 | Queiroz RML | DOI: 10.1038/s41587-018-0001-2 |
| 30528433 | The Human RNA-Binding Proteome and Its Dynamics during Translational Arrest | XRNAX & HEK293 | 2018 Dec 6 | Trendel J | DOI: 10.1016/j.cell.2018.11.004 |
| 29431736 | Capturing the interactome of newly transcribed RNA | PolyT-RICK & Hela | 2018 Feb 12 | Bao X | DOI: 10.1038/nmeth.4595 |
| 29431736 | Capturing the interactome of newly transcribed RNA | RIC & Hela | 2018 Feb 12 | Bao X | DOI: 10.1038/nmeth.4595 |
| 29431736 | Capturing the interactome of newly transcribed RNA | RICK & Hela | 2018 Feb 12 | Bao X | DOI: 10.1038/nmeth.4595 |
| 22658674 | Insights into RNA biology from an atlas of mammalian mRNA-binding proteins | RIC & Hela | 2012 May 31 | Castello A | DOI: 10.1016/j.cell.2012.04.031 |
| 30528433 | The Human RNA-Binding Proteome and Its Dynamics during Translational Arrest | XRNAX & Hela | 2018 Dec 6 | Trendel J | DOI: 10.1016/j.cell.2018.11.004 |
| 30607034 | Comprehensive identification of RNA protein interactions in any organism using orthogonal organic phase separation (OOPS) | OOPS & MCF10A | 2019 Jan 3 | Queiroz RML | DOI: 10.1038/s41587-018-0001-2 |
| 30528433 | The Human RNA-Binding Proteome and Its Dynamics during Translational Arrest | XRNAX & MCF7 | 2018 Dec 6 | Trendel J | DOI: 10.1016/j.cell.2018.11.004 |
| 30607034 | Comprehensive identification of RNA protein interactions in any organism using orthogonal organic phase separation (OOPS) | OOPS & U2OS | 2019 Jan 3 | Queiroz RML | DOI: 10.1038/s41587-018-0001-2 |
| 30352994 | Discovery of RNA-binding proteins and characterization of their dynamic responses by enhanced RNA interactome capture | RIC & Jurkat | 2018 Oct 23 | Perez-Perri JI | DOI:10.1038/s41467-018-06557-8 |
Protein-Protein Interaction (PPI)
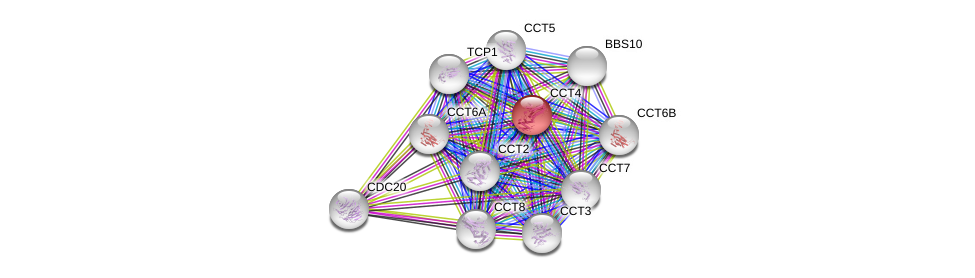
* RBP PPI network refers to all genes directly bind to RBP
Orthologs identified by RBPome
| Ensembl ID | Gene Symbol | Coverage | Identiy |
Ortholog | Gene Symbol | Coverage | Identiy |
Species |
|---|
| ENSG00000115484 | CCT4 | 97 | 64.436 | WBGene00000379 | cct-4 | 95 | 64.981 | Caenorhabditis_elegans |
|---|
| ENSG00000115484 | CCT4 | 96 | 70.213 | FBgn0032444 | CCT4 | 97 | 70.155 | Drosophila_melanogaster |
|---|
| ENSG00000115484 | CCT4 | 100 | 96.660 | ENSMUSG00000007739 | Cct4 | 100 | 96.660 | Mus_musculus |
|---|
| ENSG00000115484 | CCT4 | 96 | 59.579 | YDL143W | CCT4 | 98 | 59.579 | Saccharomyces_cerevisiae |
|---|